CAR T Cells: Expanding into Numerous Myeloma
June 12, 2017, by NCI Staff
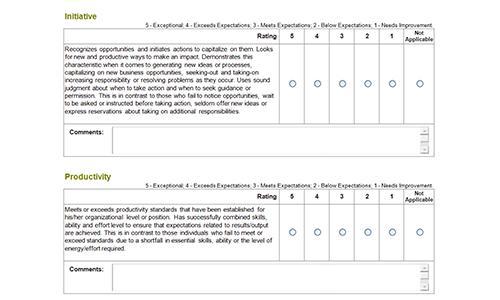
Before (left) and after (right) PET scans of a patient with numerous myeloma treated with BCMA-targeted CAR T cells.
Results from two early-phase clinical trials suggest that a form of immunotherapy that uses genetically engineered immune cells may be very effective in patients with advanced numerous myeloma.
Both trials used CAR T cells that were engineered to target a protein on myeloma cells called B-cell maturation antigen (BCMA). Albeit most patients in the trials had good responses to the treatment, including many who experienced finish remissions, the length of follow-up for most patients is still limited, researchers from both trials cautioned.
Preliminary findings from the trials were introduced this week at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. One trial was conducted in China and the other in the United States.
Serious side effects, including immune-related effects seen in other CAR T-cell trials, have been limited in the patients treated to date, reported the investigators leading the trials.
Albeit it’s still too early to determine whether these BCMA-targeted CAR T cells will eventually become standard treatments for numerous myeloma, some researchers were encouraged by the early results.
“The science behind [CAR T cells] is getting to be fairly revolutionary,” said Michael Sabel, M.D., of the University of Michigan Comprehensive Cancer Center, during a press briefing on the results of the Chinese trial. But more research is needed, Dr. Sabel continued, to validate these early findings and “to see if we can make this type of therapy accessible to more patients.”
An Emerging Form of Immunotherapy
A form of immunotherapy known as checkpoint inhibition is rapidly becoming an established part of everyday cancer care. The Food and Drug Administration (FDA) has approved checkpoint inhibitors, which are biologic drugs known as humanized monoclonal antibodies, for more types of cancer, including melanoma, lung cancer, lymphoma, and bladder cancer.
And in May, the agency approved the checkpoint inhibitor pembrolizumab (Keytruda®) for use in adults or children with advanced cancer—regardless of their type of cancer—whose tumors have one of two types of genetic alterations.
CAR T cells, on the other arm, use a patient’s own immune cells to treat their disease and are still largely in the clinical development phase.
In this form of therapy, immune cells are collected from a patient’s blood; engineered in the laboratory to produce special receptors called chimeric-antigen receptors, or CARs, that fasten to a specific target on cancer cells (an antigen); grown in the lab until they number in the billions; and then reinfused back into the patient.
Two companies have recently filed approval applications with FDA for their CAR T-cell therapies, one for lymphoma and the other for leukemia.
The CAR T-cell products under development differ from one another in several ways, including the antigen on tumor cells that they are engineered to target. Both of the CAR T-cell products that have been submitted for FDA approval, including one originally developed at NCI, target the CD19 antigen.
CAR T cells that target BCMA are relatively fresh. BCMA-targeted CAR T cells were very first tested in two thousand fourteen at NCI, and several other ongoing trials, in addition to the two reported at ASCO, are testing them.
In blood cells, the expression of BCMA is limited primarily to normal and cancerous plasma cells, explained James Kochenderfer, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research. BCMA is voiced in most patients with numerous myeloma, he added, and it’s not voiced on any nonblood cells.
“The most significant factor in determining what antigen to target with a CAR is to pick one that is not voiced on essential normal tissues,” said Dr. Kochenderfer, senior author of the US-conducted trial. Dr. Kochenderfer’s lab developed the very first BCMA-targeted CAR T cells and, in 2014, he and his colleagues at NCI conducted the very first human trial to test BCMA-targeted CAR T cells.
Many Remissions
In the Chinese trial, which is being funded by Nanjing Legend Biotech, thirty three of the thirty five patients in the examine went into accomplish remission within two months of receiving the BCMA-targeted CAR T cells, reported trial investigator Wanhong Zhao, M.D., Ph.D., of Xi’an Jiaotong University. The remaining two patients also had tumor responses, Dr. Zhao said.
Of the nineteen patients who have been followed for at least four months, fourteen have had what is called a stringent finish response (sCR)—that is, no evidence of the disease in their bone marrow or other markers of the disease in other tests—to the treatment, which the company is calling LCAR-B38M CAR T cells. Of the fourteen patients with an sCR, the five who have been followed for at least a year still have no signs of cancer, Dr. Zhao said.
Jesus G. Berdeja, M.D., of the Sarah Cannon Research Institute in Tennessee, introduced updated findings from the other trial of BCMA-targeted CAR T cells, which is being funded by bluebird bio, Inc. To date, twenty one patients have been treated on the trial. Dr. Berdeja reported on data from eighteen of these patients. (The remaining three have not been on the trial long enough to be evaluated.)
As is often done in phase I trials, different doses of the CAR T-cell product—which the company is calling bb2121—were tested, with the intention of little by little enhancing the dose if no serious side effects were seen and establishing the best dose to test in larger trials.
Only one of the very first three patients treated at the lowest dose of CAR T cells responded. Beyond this first-level dose, however, the overall response rate was 100%, with 27% of patients experiencing an sCR and the remainder having what is known as a very good partial response (based on widely used and accepted criteria).
“These patients were powerfully pretreated,” Dr. Berdeja said. The median number of prior treatments was seven, he noted; all of the patients in the trial had at least one stem cell transplant, and several have had numerous transplants.
The strong responses in patients who had been so intensely pretreated are not the only encouraging findings from the trial, Dr. Berdeja said.
“What’s so striking is that the tumor cells are killed so quickly that all the other parameters [of treatment response] lag behind,” he said.
Manageable Side Effects
All patients undergoing CAR T-cell therapy are closely monitored for a known side effect called cytokine release syndrome (CRS), a massive storm of immune signaling cells called cytokines that can be fatal.
Minor cases of CRS were common in both trials, the researchers reported, but were generally brief lived and managed with standard therapies.
Several patients in both trials experienced severe CRS following treatment, which was treated successfully with the drug tocilizumab (Actemra®).
No patients in either trial experienced neurotoxicity, another serious side effect that has been seen in several other trials of CAR T cells and, in one case, led a company to discontinue development of its lead CAR T cell treatment.
Differences in the Therapies, Trials
Unlike most other trials of CAR T cells, including the bluebird trial, patients in the Chinese trial received three smaller doses of the cells rather than a single large dose. Patients received a relatively puny dose on their very first day of therapy, and then two progressively larger doses on days two and 6.
This was done largely for safety reasons, explained Frank Fan, Ph.D., the chief scientific officer for Nanjing Legend. In some cases, if patients were experiencing CRS or any other potentially serious side effect after the 2nd dose, the third dose was not given, Dr. Fan said.
The receptor on the LCAR-B38M CAR T cells is also different from those on most other CAR T cells in development, he continued, in that it can link to two different parts of the same antigen. This difference, he believes, may improve its capability to truss to and kill tumor cells.
Interpreting the significance of the Chinese trial results is difficult because of some significant missing details, said Marcela Maus, M.D., Ph.D., director of Cellular Immunotherapy at the Massachusetts General Hospital Cancer Center.
During an abstract discussion session at the meeting where the trial results were introduced, Dr. Maus said more information is still needed on the extent and types of prior therapies received by patients, the criteria used to determine the treatment responses, and the composition of the T cells used in the therapy administered to patients.
In the bluebird trial, not only had all of the patients been intensely pretreated, Dr. Berdeja said, but almost 30% of patients were resistant to all of the major drug classes used to treat numerous myeloma.
Next Steps
The researchers leading the Nanjing Legend trial are continuing to enroll patients in their trial and are filing the regulatory paperwork with FDA to launch a similar trial in the United States early next year, Dr. Fan said.
The bluebird trial will now proceed to what is known as the dose-expansion stage, Dr. Berdeja explained, using the dose established as most safe and effective in the trial’s initial dose-escalation stage.
CAR T-Cell Therapy for Numerous Myeloma – National Cancer Institute
CAR T Cells: Expanding into Numerous Myeloma
June 12, 2017, by NCI Staff
Before (left) and after (right) PET scans of a patient with numerous myeloma treated with BCMA-targeted CAR T cells.
Results from two early-phase clinical trials suggest that a form of immunotherapy that uses genetically engineered immune cells may be very effective in patients with advanced numerous myeloma.
Both trials used CAR T cells that were engineered to target a protein on myeloma cells called B-cell maturation antigen (BCMA). Albeit most patients in the trials had good responses to the treatment, including many who experienced accomplish remissions, the length of follow-up for most patients is still limited, researchers from both trials cautioned.
Preliminary findings from the trials were introduced this week at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. One trial was conducted in China and the other in the United States.
Serious side effects, including immune-related effects seen in other CAR T-cell trials, have been limited in the patients treated to date, reported the investigators leading the trials.
Albeit it’s still too early to determine whether these BCMA-targeted CAR T cells will eventually become standard treatments for numerous myeloma, some researchers were encouraged by the early results.
“The science behind [CAR T cells] is getting to be fairly revolutionary,” said Michael Sabel, M.D., of the University of Michigan Comprehensive Cancer Center, during a press briefing on the results of the Chinese trial. But more research is needed, Dr. Sabel continued, to validate these early findings and “to see if we can make this type of therapy accessible to more patients.”
An Emerging Form of Immunotherapy
A form of immunotherapy known as checkpoint inhibition is rapidly becoming an established part of everyday cancer care. The Food and Drug Administration (FDA) has approved checkpoint inhibitors, which are biologic drugs known as humanized monoclonal antibodies, for more types of cancer, including melanoma, lung cancer, lymphoma, and bladder cancer.
And in May, the agency approved the checkpoint inhibitor pembrolizumab (Keytruda®) for use in adults or children with advanced cancer—regardless of their type of cancer—whose tumors have one of two types of genetic alterations.
CAR T cells, on the other arm, use a patient’s own immune cells to treat their disease and are still largely in the clinical development phase.
In this form of therapy, immune cells are collected from a patient’s blood; engineered in the laboratory to produce special receptors called chimeric-antigen receptors, or CARs, that link to a specific target on cancer cells (an antigen); grown in the lab until they number in the billions; and then reinfused back into the patient.
Two companies have recently filed approval applications with FDA for their CAR T-cell therapies, one for lymphoma and the other for leukemia.
The CAR T-cell products under development differ from one another in several ways, including the antigen on tumor cells that they are engineered to target. Both of the CAR T-cell products that have been submitted for FDA approval, including one primarily developed at NCI, target the CD19 antigen.
CAR T cells that target BCMA are relatively fresh. BCMA-targeted CAR T cells were very first tested in two thousand fourteen at NCI, and several other ongoing trials, in addition to the two reported at ASCO, are testing them.
In blood cells, the expression of BCMA is limited primarily to normal and cancerous plasma cells, explained James Kochenderfer, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research. BCMA is voiced in most patients with numerous myeloma, he added, and it’s not voiced on any nonblood cells.
“The most significant factor in determining what antigen to target with a CAR is to pick one that is not voiced on essential normal tissues,” said Dr. Kochenderfer, senior author of the US-conducted trial. Dr. Kochenderfer’s lab developed the very first BCMA-targeted CAR T cells and, in 2014, he and his colleagues at NCI conducted the very first human trial to test BCMA-targeted CAR T cells.
Many Remissions
In the Chinese trial, which is being funded by Nanjing Legend Biotech, thirty three of the thirty five patients in the investigate went into accomplish remission within two months of receiving the BCMA-targeted CAR T cells, reported trial investigator Wanhong Zhao, M.D., Ph.D., of Xi’an Jiaotong University. The remaining two patients also had tumor responses, Dr. Zhao said.
Of the nineteen patients who have been followed for at least four months, fourteen have had what is called a stringent finish response (sCR)—that is, no evidence of the disease in their bone marrow or other markers of the disease in other tests—to the treatment, which the company is calling LCAR-B38M CAR T cells. Of the fourteen patients with an sCR, the five who have been followed for at least a year still have no signs of cancer, Dr. Zhao said.
Jesus G. Berdeja, M.D., of the Sarah Cannon Research Institute in Tennessee, introduced updated findings from the other trial of BCMA-targeted CAR T cells, which is being funded by bluebird bio, Inc. To date, twenty one patients have been treated on the trial. Dr. Berdeja reported on data from eighteen of these patients. (The remaining three have not been on the trial long enough to be evaluated.)
As is often done in phase I trials, different doses of the CAR T-cell product—which the company is calling bb2121—were tested, with the intention of step by step enhancing the dose if no serious side effects were seen and establishing the best dose to test in larger trials.
Only one of the very first three patients treated at the lowest dose of CAR T cells responded. Beyond this first-level dose, however, the overall response rate was 100%, with 27% of patients experiencing an sCR and the remainder having what is known as a very good partial response (based on widely used and accepted criteria).
“These patients were strenuously pretreated,” Dr. Berdeja said. The median number of prior treatments was seven, he noted; all of the patients in the trial had at least one stem cell transplant, and several have had numerous transplants.
The strong responses in patients who had been so intensely pretreated are not the only encouraging findings from the trial, Dr. Berdeja said.
“What’s so striking is that the tumor cells are killed so quickly that all the other parameters [of treatment response] lag behind,” he said.
Manageable Side Effects
All patients undergoing CAR T-cell therapy are closely monitored for a known side effect called cytokine release syndrome (CRS), a massive storm of immune signaling cells called cytokines that can be fatal.
Minor cases of CRS were common in both trials, the researchers reported, but were generally brief lived and managed with standard therapies.
Several patients in both trials experienced severe CRS following treatment, which was treated successfully with the drug tocilizumab (Actemra®).
No patients in either trial experienced neurotoxicity, another serious side effect that has been seen in several other trials of CAR T cells and, in one case, led a company to discontinue development of its lead CAR T cell treatment.
Differences in the Therapies, Trials
Unlike most other trials of CAR T cells, including the bluebird trial, patients in the Chinese trial received three smaller doses of the cells rather than a single large dose. Patients received a relatively puny dose on their very first day of therapy, and then two progressively larger doses on days two and 6.
This was done largely for safety reasons, explained Frank Fan, Ph.D., the chief scientific officer for Nanjing Legend. In some cases, if patients were experiencing CRS or any other potentially serious side effect after the 2nd dose, the third dose was not given, Dr. Fan said.
The receptor on the LCAR-B38M CAR T cells is also different from those on most other CAR T cells in development, he continued, in that it can link to two different parts of the same antigen. This difference, he believes, may improve its capability to truss to and kill tumor cells.
Interpreting the significance of the Chinese trial results is difficult because of some significant missing details, said Marcela Maus, M.D., Ph.D., director of Cellular Immunotherapy at the Massachusetts General Hospital Cancer Center.
During an abstract discussion session at the meeting where the trial results were introduced, Dr. Maus said more information is still needed on the extent and types of prior therapies received by patients, the criteria used to determine the treatment responses, and the composition of the T cells used in the therapy administered to patients.
In the bluebird trial, not only had all of the patients been strongly pretreated, Dr. Berdeja said, but almost 30% of patients were resistant to all of the major drug classes used to treat numerous myeloma.
Next Steps
The researchers leading the Nanjing Legend trial are continuing to enroll patients in their trial and are filing the regulatory paperwork with FDA to launch a similar trial in the United States early next year, Dr. Fan said.
The bluebird trial will now proceed to what is known as the dose-expansion stage, Dr. Berdeja explained, using the dose established as most safe and effective in the trial’s initial dose-escalation stage.
CAR T-Cell Therapy for Numerous Myeloma – National Cancer Institute
CAR T Cells: Expanding into Numerous Myeloma
June 12, 2017, by NCI Staff
Before (left) and after (right) PET scans of a patient with numerous myeloma treated with BCMA-targeted CAR T cells.
Results from two early-phase clinical trials suggest that a form of immunotherapy that uses genetically engineered immune cells may be very effective in patients with advanced numerous myeloma.
Both trials used CAR T cells that were engineered to target a protein on myeloma cells called B-cell maturation antigen (BCMA). Albeit most patients in the trials had good responses to the treatment, including many who experienced finish remissions, the length of follow-up for most patients is still limited, researchers from both trials cautioned.
Preliminary findings from the trials were introduced this week at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. One trial was conducted in China and the other in the United States.
Serious side effects, including immune-related effects seen in other CAR T-cell trials, have been limited in the patients treated to date, reported the investigators leading the trials.
Albeit it’s still too early to determine whether these BCMA-targeted CAR T cells will eventually become standard treatments for numerous myeloma, some researchers were encouraged by the early results.
“The science behind [CAR T cells] is getting to be fairly revolutionary,” said Michael Sabel, M.D., of the University of Michigan Comprehensive Cancer Center, during a press briefing on the results of the Chinese trial. But more research is needed, Dr. Sabel continued, to validate these early findings and “to see if we can make this type of therapy accessible to more patients.”
An Emerging Form of Immunotherapy
A form of immunotherapy known as checkpoint inhibition is rapidly becoming an established part of everyday cancer care. The Food and Drug Administration (FDA) has approved checkpoint inhibitors, which are biologic drugs known as humanized monoclonal antibodies, for more types of cancer, including melanoma, lung cancer, lymphoma, and bladder cancer.
And in May, the agency approved the checkpoint inhibitor pembrolizumab (Keytruda®) for use in adults or children with advanced cancer—regardless of their type of cancer—whose tumors have one of two types of genetic alterations.
CAR T cells, on the other forearm, use a patient’s own immune cells to treat their disease and are still largely in the clinical development phase.
In this form of therapy, immune cells are collected from a patient’s blood; engineered in the laboratory to produce special receptors called chimeric-antigen receptors, or CARs, that fasten to a specific target on cancer cells (an antigen); grown in the lab until they number in the billions; and then reinfused back into the patient.
Two companies have recently filed approval applications with FDA for their CAR T-cell therapies, one for lymphoma and the other for leukemia.
The CAR T-cell products under development differ from one another in several ways, including the antigen on tumor cells that they are engineered to target. Both of the CAR T-cell products that have been submitted for FDA approval, including one primarily developed at NCI, target the CD19 antigen.
CAR T cells that target BCMA are relatively fresh. BCMA-targeted CAR T cells were very first tested in two thousand fourteen at NCI, and several other ongoing trials, in addition to the two reported at ASCO, are testing them.
In blood cells, the expression of BCMA is limited primarily to normal and cancerous plasma cells, explained James Kochenderfer, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research. BCMA is voiced in most patients with numerous myeloma, he added, and it’s not voiced on any nonblood cells.
“The most significant factor in determining what antigen to target with a CAR is to pick one that is not voiced on essential normal tissues,” said Dr. Kochenderfer, senior author of the US-conducted trial. Dr. Kochenderfer’s lab developed the very first BCMA-targeted CAR T cells and, in 2014, he and his colleagues at NCI conducted the very first human trial to test BCMA-targeted CAR T cells.
Many Remissions
In the Chinese trial, which is being funded by Nanjing Legend Biotech, thirty three of the thirty five patients in the investigate went into accomplish remission within two months of receiving the BCMA-targeted CAR T cells, reported trial investigator Wanhong Zhao, M.D., Ph.D., of Xi’an Jiaotong University. The remaining two patients also had tumor responses, Dr. Zhao said.
Of the nineteen patients who have been followed for at least four months, fourteen have had what is called a stringent finish response (sCR)—that is, no evidence of the disease in their bone marrow or other markers of the disease in other tests—to the treatment, which the company is calling LCAR-B38M CAR T cells. Of the fourteen patients with an sCR, the five who have been followed for at least a year still have no signs of cancer, Dr. Zhao said.
Jesus G. Berdeja, M.D., of the Sarah Cannon Research Institute in Tennessee, introduced updated findings from the other trial of BCMA-targeted CAR T cells, which is being funded by bluebird bio, Inc. To date, twenty one patients have been treated on the trial. Dr. Berdeja reported on data from eighteen of these patients. (The remaining three have not been on the trial long enough to be evaluated.)
As is often done in phase I trials, different doses of the CAR T-cell product—which the company is calling bb2121—were tested, with the intention of step by step enhancing the dose if no serious side effects were seen and establishing the best dose to test in larger trials.
Only one of the very first three patients treated at the lowest dose of CAR T cells responded. Beyond this first-level dose, however, the overall response rate was 100%, with 27% of patients experiencing an sCR and the remainder having what is known as a very good partial response (based on widely used and accepted criteria).
“These patients were strongly pretreated,” Dr. Berdeja said. The median number of prior treatments was seven, he noted; all of the patients in the trial had at least one stem cell transplant, and several have had numerous transplants.
The strong responses in patients who had been so intensely pretreated are not the only encouraging findings from the trial, Dr. Berdeja said.
“What’s so striking is that the tumor cells are killed so quickly that all the other parameters [of treatment response] lag behind,” he said.
Manageable Side Effects
All patients undergoing CAR T-cell therapy are closely monitored for a known side effect called cytokine release syndrome (CRS), a massive storm of immune signaling cells called cytokines that can be fatal.
Minor cases of CRS were common in both trials, the researchers reported, but were generally brief lived and managed with standard therapies.
Several patients in both trials experienced severe CRS following treatment, which was treated successfully with the drug tocilizumab (Actemra®).
No patients in either trial experienced neurotoxicity, another serious side effect that has been seen in several other trials of CAR T cells and, in one case, led a company to discontinue development of its lead CAR T cell treatment.
Differences in the Therapies, Trials
Unlike most other trials of CAR T cells, including the bluebird trial, patients in the Chinese trial received three smaller doses of the cells rather than a single large dose. Patients received a relatively puny dose on their very first day of therapy, and then two progressively larger doses on days two and 6.
This was done largely for safety reasons, explained Frank Fan, Ph.D., the chief scientific officer for Nanjing Legend. In some cases, if patients were experiencing CRS or any other potentially serious side effect after the 2nd dose, the third dose was not given, Dr. Fan said.
The receptor on the LCAR-B38M CAR T cells is also different from those on most other CAR T cells in development, he continued, in that it can fasten to two different parts of the same antigen. This difference, he believes, may improve its capability to truss to and kill tumor cells.
Interpreting the significance of the Chinese trial results is difficult because of some significant missing details, said Marcela Maus, M.D., Ph.D., director of Cellular Immunotherapy at the Massachusetts General Hospital Cancer Center.
During an abstract discussion session at the meeting where the trial results were introduced, Dr. Maus said more information is still needed on the extent and types of prior therapies received by patients, the criteria used to determine the treatment responses, and the composition of the T cells used in the therapy administered to patients.
In the bluebird trial, not only had all of the patients been strenuously pretreated, Dr. Berdeja said, but almost 30% of patients were resistant to all of the major drug classes used to treat numerous myeloma.
Next Steps
The researchers leading the Nanjing Legend trial are continuing to enroll patients in their trial and are filing the regulatory paperwork with FDA to launch a similar trial in the United States early next year, Dr. Fan said.
The bluebird trial will now proceed to what is known as the dose-expansion stage, Dr. Berdeja explained, using the dose established as most safe and effective in the trial’s initial dose-escalation stage.
CAR T-Cell Therapy for Numerous Myeloma – National Cancer Institute
CAR T Cells: Expanding into Numerous Myeloma
June 12, 2017, by NCI Staff
Before (left) and after (right) PET scans of a patient with numerous myeloma treated with BCMA-targeted CAR T cells.
Results from two early-phase clinical trials suggest that a form of immunotherapy that uses genetically engineered immune cells may be very effective in patients with advanced numerous myeloma.
Both trials used CAR T cells that were engineered to target a protein on myeloma cells called B-cell maturation antigen (BCMA). Albeit most patients in the trials had good responses to the treatment, including many who experienced finish remissions, the length of follow-up for most patients is still limited, researchers from both trials cautioned.
Preliminary findings from the trials were introduced this week at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. One trial was conducted in China and the other in the United States.
Serious side effects, including immune-related effects seen in other CAR T-cell trials, have been limited in the patients treated to date, reported the investigators leading the trials.
Albeit it’s still too early to determine whether these BCMA-targeted CAR T cells will eventually become standard treatments for numerous myeloma, some researchers were encouraged by the early results.
“The science behind [CAR T cells] is getting to be fairly revolutionary,” said Michael Sabel, M.D., of the University of Michigan Comprehensive Cancer Center, during a press briefing on the results of the Chinese trial. But more research is needed, Dr. Sabel continued, to validate these early findings and “to see if we can make this type of therapy accessible to more patients.”
An Emerging Form of Immunotherapy
A form of immunotherapy known as checkpoint inhibition is rapidly becoming an established part of everyday cancer care. The Food and Drug Administration (FDA) has approved checkpoint inhibitors, which are biologic drugs known as humanized monoclonal antibodies, for more types of cancer, including melanoma, lung cancer, lymphoma, and bladder cancer.
And in May, the agency approved the checkpoint inhibitor pembrolizumab (Keytruda®) for use in adults or children with advanced cancer—regardless of their type of cancer—whose tumors have one of two types of genetic alterations.
CAR T cells, on the other arm, use a patient’s own immune cells to treat their disease and are still largely in the clinical development phase.
In this form of therapy, immune cells are collected from a patient’s blood; engineered in the laboratory to produce special receptors called chimeric-antigen receptors, or CARs, that fasten to a specific target on cancer cells (an antigen); grown in the lab until they number in the billions; and then reinfused back into the patient.
Two companies have recently filed approval applications with FDA for their CAR T-cell therapies, one for lymphoma and the other for leukemia.
The CAR T-cell products under development differ from one another in several ways, including the antigen on tumor cells that they are engineered to target. Both of the CAR T-cell products that have been submitted for FDA approval, including one primarily developed at NCI, target the CD19 antigen.
CAR T cells that target BCMA are relatively fresh. BCMA-targeted CAR T cells were very first tested in two thousand fourteen at NCI, and several other ongoing trials, in addition to the two reported at ASCO, are testing them.
In blood cells, the expression of BCMA is limited primarily to normal and cancerous plasma cells, explained James Kochenderfer, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research. BCMA is voiced in most patients with numerous myeloma, he added, and it’s not voiced on any nonblood cells.
“The most significant factor in determining what antigen to target with a CAR is to pick one that is not voiced on essential normal tissues,” said Dr. Kochenderfer, senior author of the US-conducted trial. Dr. Kochenderfer’s lab developed the very first BCMA-targeted CAR T cells and, in 2014, he and his colleagues at NCI conducted the very first human trial to test BCMA-targeted CAR T cells.
Many Remissions
In the Chinese trial, which is being funded by Nanjing Legend Biotech, thirty three of the thirty five patients in the examine went into finish remission within two months of receiving the BCMA-targeted CAR T cells, reported trial investigator Wanhong Zhao, M.D., Ph.D., of Xi’an Jiaotong University. The remaining two patients also had tumor responses, Dr. Zhao said.
Of the nineteen patients who have been followed for at least four months, fourteen have had what is called a stringent accomplish response (sCR)—that is, no evidence of the disease in their bone marrow or other markers of the disease in other tests—to the treatment, which the company is calling LCAR-B38M CAR T cells. Of the fourteen patients with an sCR, the five who have been followed for at least a year still have no signs of cancer, Dr. Zhao said.
Jesus G. Berdeja, M.D., of the Sarah Cannon Research Institute in Tennessee, introduced updated findings from the other trial of BCMA-targeted CAR T cells, which is being funded by bluebird bio, Inc. To date, twenty one patients have been treated on the trial. Dr. Berdeja reported on data from eighteen of these patients. (The remaining three have not been on the trial long enough to be evaluated.)
As is often done in phase I trials, different doses of the CAR T-cell product—which the company is calling bb2121—were tested, with the intention of little by little enlargening the dose if no serious side effects were seen and establishing the best dose to test in larger trials.
Only one of the very first three patients treated at the lowest dose of CAR T cells responded. Beyond this first-level dose, however, the overall response rate was 100%, with 27% of patients experiencing an sCR and the remainder having what is known as a very good partial response (based on widely used and accepted criteria).
“These patients were powerfully pretreated,” Dr. Berdeja said. The median number of prior treatments was seven, he noted; all of the patients in the trial had at least one stem cell transplant, and several have had numerous transplants.
The strong responses in patients who had been so strenuously pretreated are not the only encouraging findings from the trial, Dr. Berdeja said.
“What’s so striking is that the tumor cells are killed so quickly that all the other parameters [of treatment response] lag behind,” he said.
Manageable Side Effects
All patients undergoing CAR T-cell therapy are closely monitored for a known side effect called cytokine release syndrome (CRS), a massive storm of immune signaling cells called cytokines that can be fatal.
Minor cases of CRS were common in both trials, the researchers reported, but were generally brief lived and managed with standard therapies.
Several patients in both trials experienced severe CRS following treatment, which was treated successfully with the drug tocilizumab (Actemra®).
No patients in either trial experienced neurotoxicity, another serious side effect that has been seen in several other trials of CAR T cells and, in one case, led a company to discontinue development of its lead CAR T cell treatment.
Differences in the Therapies, Trials
Unlike most other trials of CAR T cells, including the bluebird trial, patients in the Chinese trial received three smaller doses of the cells rather than a single large dose. Patients received a relatively petite dose on their very first day of therapy, and then two progressively larger doses on days two and 6.
This was done largely for safety reasons, explained Frank Fan, Ph.D., the chief scientific officer for Nanjing Legend. In some cases, if patients were experiencing CRS or any other potentially serious side effect after the 2nd dose, the third dose was not given, Dr. Fan said.
The receptor on the LCAR-B38M CAR T cells is also different from those on most other CAR T cells in development, he continued, in that it can fasten to two different parts of the same antigen. This difference, he believes, may improve its capability to tie to and kill tumor cells.
Interpreting the significance of the Chinese trial results is difficult because of some significant missing details, said Marcela Maus, M.D., Ph.D., director of Cellular Immunotherapy at the Massachusetts General Hospital Cancer Center.
During an abstract discussion session at the meeting where the trial results were introduced, Dr. Maus said more information is still needed on the extent and types of prior therapies received by patients, the criteria used to determine the treatment responses, and the composition of the T cells used in the therapy administered to patients.
In the bluebird trial, not only had all of the patients been strenuously pretreated, Dr. Berdeja said, but almost 30% of patients were resistant to all of the major drug classes used to treat numerous myeloma.
Next Steps
The researchers leading the Nanjing Legend trial are continuing to enroll patients in their trial and are filing the regulatory paperwork with FDA to launch a similar trial in the United States early next year, Dr. Fan said.
The bluebird trial will now proceed to what is known as the dose-expansion stage, Dr. Berdeja explained, using the dose established as most safe and effective in the trial’s initial dose-escalation stage.
CAR T-Cell Therapy for Numerous Myeloma – National Cancer Institute
CAR T Cells: Expanding into Numerous Myeloma
June 12, 2017, by NCI Staff
Before (left) and after (right) PET scans of a patient with numerous myeloma treated with BCMA-targeted CAR T cells.
Results from two early-phase clinical trials suggest that a form of immunotherapy that uses genetically engineered immune cells may be very effective in patients with advanced numerous myeloma.
Both trials used CAR T cells that were engineered to target a protein on myeloma cells called B-cell maturation antigen (BCMA). Albeit most patients in the trials had good responses to the treatment, including many who experienced accomplish remissions, the length of follow-up for most patients is still limited, researchers from both trials cautioned.
Preliminary findings from the trials were introduced this week at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. One trial was conducted in China and the other in the United States.
Serious side effects, including immune-related effects seen in other CAR T-cell trials, have been limited in the patients treated to date, reported the investigators leading the trials.
Albeit it’s still too early to determine whether these BCMA-targeted CAR T cells will eventually become standard treatments for numerous myeloma, some researchers were encouraged by the early results.
“The science behind [CAR T cells] is getting to be fairly revolutionary,” said Michael Sabel, M.D., of the University of Michigan Comprehensive Cancer Center, during a press briefing on the results of the Chinese trial. But more research is needed, Dr. Sabel continued, to validate these early findings and “to see if we can make this type of therapy accessible to more patients.”
An Emerging Form of Immunotherapy
A form of immunotherapy known as checkpoint inhibition is rapidly becoming an established part of everyday cancer care. The Food and Drug Administration (FDA) has approved checkpoint inhibitors, which are biologic drugs known as humanized monoclonal antibodies, for more types of cancer, including melanoma, lung cancer, lymphoma, and bladder cancer.
And in May, the agency approved the checkpoint inhibitor pembrolizumab (Keytruda®) for use in adults or children with advanced cancer—regardless of their type of cancer—whose tumors have one of two types of genetic alterations.
CAR T cells, on the other forearm, use a patient’s own immune cells to treat their disease and are still largely in the clinical development phase.
In this form of therapy, immune cells are collected from a patient’s blood; engineered in the laboratory to produce special receptors called chimeric-antigen receptors, or CARs, that fasten to a specific target on cancer cells (an antigen); grown in the lab until they number in the billions; and then reinfused back into the patient.
Two companies have recently filed approval applications with FDA for their CAR T-cell therapies, one for lymphoma and the other for leukemia.
The CAR T-cell products under development differ from one another in several ways, including the antigen on tumor cells that they are engineered to target. Both of the CAR T-cell products that have been submitted for FDA approval, including one originally developed at NCI, target the CD19 antigen.
CAR T cells that target BCMA are relatively fresh. BCMA-targeted CAR T cells were very first tested in two thousand fourteen at NCI, and several other ongoing trials, in addition to the two reported at ASCO, are testing them.
In blood cells, the expression of BCMA is limited primarily to normal and cancerous plasma cells, explained James Kochenderfer, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research. BCMA is voiced in most patients with numerous myeloma, he added, and it’s not voiced on any nonblood cells.
“The most significant factor in determining what antigen to target with a CAR is to pick one that is not voiced on essential normal tissues,” said Dr. Kochenderfer, senior author of the US-conducted trial. Dr. Kochenderfer’s lab developed the very first BCMA-targeted CAR T cells and, in 2014, he and his colleagues at NCI conducted the very first human trial to test BCMA-targeted CAR T cells.
Many Remissions
In the Chinese trial, which is being funded by Nanjing Legend Biotech, thirty three of the thirty five patients in the explore went into finish remission within two months of receiving the BCMA-targeted CAR T cells, reported trial investigator Wanhong Zhao, M.D., Ph.D., of Xi’an Jiaotong University. The remaining two patients also had tumor responses, Dr. Zhao said.
Of the nineteen patients who have been followed for at least four months, fourteen have had what is called a stringent finish response (sCR)—that is, no evidence of the disease in their bone marrow or other markers of the disease in other tests—to the treatment, which the company is calling LCAR-B38M CAR T cells. Of the fourteen patients with an sCR, the five who have been followed for at least a year still have no signs of cancer, Dr. Zhao said.
Jesus G. Berdeja, M.D., of the Sarah Cannon Research Institute in Tennessee, introduced updated findings from the other trial of BCMA-targeted CAR T cells, which is being funded by bluebird bio, Inc. To date, twenty one patients have been treated on the trial. Dr. Berdeja reported on data from eighteen of these patients. (The remaining three have not been on the trial long enough to be evaluated.)
As is often done in phase I trials, different doses of the CAR T-cell product—which the company is calling bb2121—were tested, with the intention of little by little enlargening the dose if no serious side effects were seen and establishing the best dose to test in larger trials.
Only one of the very first three patients treated at the lowest dose of CAR T cells responded. Beyond this first-level dose, however, the overall response rate was 100%, with 27% of patients experiencing an sCR and the remainder having what is known as a very good partial response (based on widely used and accepted criteria).
“These patients were strongly pretreated,” Dr. Berdeja said. The median number of prior treatments was seven, he noted; all of the patients in the trial had at least one stem cell transplant, and several have had numerous transplants.
The strong responses in patients who had been so powerfully pretreated are not the only encouraging findings from the trial, Dr. Berdeja said.
“What’s so striking is that the tumor cells are killed so quickly that all the other parameters [of treatment response] lag behind,” he said.
Manageable Side Effects
All patients undergoing CAR T-cell therapy are closely monitored for a known side effect called cytokine release syndrome (CRS), a massive storm of immune signaling cells called cytokines that can be fatal.
Minor cases of CRS were common in both trials, the researchers reported, but were generally brief lived and managed with standard therapies.
Several patients in both trials experienced severe CRS following treatment, which was treated successfully with the drug tocilizumab (Actemra®).
No patients in either trial experienced neurotoxicity, another serious side effect that has been seen in several other trials of CAR T cells and, in one case, led a company to discontinue development of its lead CAR T cell treatment.
Differences in the Therapies, Trials
Unlike most other trials of CAR T cells, including the bluebird trial, patients in the Chinese trial received three smaller doses of the cells rather than a single large dose. Patients received a relatively petite dose on their very first day of therapy, and then two progressively larger doses on days two and 6.
This was done largely for safety reasons, explained Frank Fan, Ph.D., the chief scientific officer for Nanjing Legend. In some cases, if patients were experiencing CRS or any other potentially serious side effect after the 2nd dose, the third dose was not given, Dr. Fan said.
The receptor on the LCAR-B38M CAR T cells is also different from those on most other CAR T cells in development, he continued, in that it can link to two different parts of the same antigen. This difference, he believes, may improve its capability to tie to and kill tumor cells.
Interpreting the significance of the Chinese trial results is difficult because of some significant missing details, said Marcela Maus, M.D., Ph.D., director of Cellular Immunotherapy at the Massachusetts General Hospital Cancer Center.
During an abstract discussion session at the meeting where the trial results were introduced, Dr. Maus said more information is still needed on the extent and types of prior therapies received by patients, the criteria used to determine the treatment responses, and the composition of the T cells used in the therapy administered to patients.
In the bluebird trial, not only had all of the patients been strenuously pretreated, Dr. Berdeja said, but almost 30% of patients were resistant to all of the major drug classes used to treat numerous myeloma.
Next Steps
The researchers leading the Nanjing Legend trial are continuing to enroll patients in their trial and are filing the regulatory paperwork with FDA to launch a similar trial in the United States early next year, Dr. Fan said.
The bluebird trial will now proceed to what is known as the dose-expansion stage, Dr. Berdeja explained, using the dose established as most safe and effective in the trial’s initial dose-escalation stage.
CAR T-Cell Therapy for Numerous Myeloma – National Cancer Institute
CAR T Cells: Expanding into Numerous Myeloma
June 12, 2017, by NCI Staff
Before (left) and after (right) PET scans of a patient with numerous myeloma treated with BCMA-targeted CAR T cells.
Results from two early-phase clinical trials suggest that a form of immunotherapy that uses genetically engineered immune cells may be very effective in patients with advanced numerous myeloma.
Both trials used CAR T cells that were engineered to target a protein on myeloma cells called B-cell maturation antigen (BCMA). Albeit most patients in the trials had good responses to the treatment, including many who experienced finish remissions, the length of follow-up for most patients is still limited, researchers from both trials cautioned.
Preliminary findings from the trials were introduced this week at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. One trial was conducted in China and the other in the United States.
Serious side effects, including immune-related effects seen in other CAR T-cell trials, have been limited in the patients treated to date, reported the investigators leading the trials.
Albeit it’s still too early to determine whether these BCMA-targeted CAR T cells will eventually become standard treatments for numerous myeloma, some researchers were encouraged by the early results.
“The science behind [CAR T cells] is getting to be fairly revolutionary,” said Michael Sabel, M.D., of the University of Michigan Comprehensive Cancer Center, during a press briefing on the results of the Chinese trial. But more research is needed, Dr. Sabel continued, to validate these early findings and “to see if we can make this type of therapy accessible to more patients.”
An Emerging Form of Immunotherapy
A form of immunotherapy known as checkpoint inhibition is rapidly becoming an established part of everyday cancer care. The Food and Drug Administration (FDA) has approved checkpoint inhibitors, which are biologic drugs known as humanized monoclonal antibodies, for more types of cancer, including melanoma, lung cancer, lymphoma, and bladder cancer.
And in May, the agency approved the checkpoint inhibitor pembrolizumab (Keytruda®) for use in adults or children with advanced cancer—regardless of their type of cancer—whose tumors have one of two types of genetic alterations.
CAR T cells, on the other arm, use a patient’s own immune cells to treat their disease and are still largely in the clinical development phase.
In this form of therapy, immune cells are collected from a patient’s blood; engineered in the laboratory to produce special receptors called chimeric-antigen receptors, or CARs, that fasten to a specific target on cancer cells (an antigen); grown in the lab until they number in the billions; and then reinfused back into the patient.
Two companies have recently filed approval applications with FDA for their CAR T-cell therapies, one for lymphoma and the other for leukemia.
The CAR T-cell products under development differ from one another in several ways, including the antigen on tumor cells that they are engineered to target. Both of the CAR T-cell products that have been submitted for FDA approval, including one originally developed at NCI, target the CD19 antigen.
CAR T cells that target BCMA are relatively fresh. BCMA-targeted CAR T cells were very first tested in two thousand fourteen at NCI, and several other ongoing trials, in addition to the two reported at ASCO, are testing them.
In blood cells, the expression of BCMA is limited primarily to normal and cancerous plasma cells, explained James Kochenderfer, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research. BCMA is voiced in most patients with numerous myeloma, he added, and it’s not voiced on any nonblood cells.
“The most significant factor in determining what antigen to target with a CAR is to pick one that is not voiced on essential normal tissues,” said Dr. Kochenderfer, senior author of the US-conducted trial. Dr. Kochenderfer’s lab developed the very first BCMA-targeted CAR T cells and, in 2014, he and his colleagues at NCI conducted the very first human trial to test BCMA-targeted CAR T cells.
Many Remissions
In the Chinese trial, which is being funded by Nanjing Legend Biotech, thirty three of the thirty five patients in the explore went into finish remission within two months of receiving the BCMA-targeted CAR T cells, reported trial investigator Wanhong Zhao, M.D., Ph.D., of Xi’an Jiaotong University. The remaining two patients also had tumor responses, Dr. Zhao said.
Of the nineteen patients who have been followed for at least four months, fourteen have had what is called a stringent finish response (sCR)—that is, no evidence of the disease in their bone marrow or other markers of the disease in other tests—to the treatment, which the company is calling LCAR-B38M CAR T cells. Of the fourteen patients with an sCR, the five who have been followed for at least a year still have no signs of cancer, Dr. Zhao said.
Jesus G. Berdeja, M.D., of the Sarah Cannon Research Institute in Tennessee, introduced updated findings from the other trial of BCMA-targeted CAR T cells, which is being funded by bluebird bio, Inc. To date, twenty one patients have been treated on the trial. Dr. Berdeja reported on data from eighteen of these patients. (The remaining three have not been on the trial long enough to be evaluated.)
As is often done in phase I trials, different doses of the CAR T-cell product—which the company is calling bb2121—were tested, with the intention of little by little enhancing the dose if no serious side effects were seen and establishing the best dose to test in larger trials.
Only one of the very first three patients treated at the lowest dose of CAR T cells responded. Beyond this first-level dose, however, the overall response rate was 100%, with 27% of patients experiencing an sCR and the remainder having what is known as a very good partial response (based on widely used and accepted criteria).
“These patients were strenuously pretreated,” Dr. Berdeja said. The median number of prior treatments was seven, he noted; all of the patients in the trial had at least one stem cell transplant, and several have had numerous transplants.
The strong responses in patients who had been so strenuously pretreated are not the only encouraging findings from the trial, Dr. Berdeja said.
“What’s so striking is that the tumor cells are killed so quickly that all the other parameters [of treatment response] lag behind,” he said.
Manageable Side Effects
All patients undergoing CAR T-cell therapy are closely monitored for a known side effect called cytokine release syndrome (CRS), a massive storm of immune signaling cells called cytokines that can be fatal.
Minor cases of CRS were common in both trials, the researchers reported, but were generally brief lived and managed with standard therapies.
Several patients in both trials experienced severe CRS following treatment, which was treated successfully with the drug tocilizumab (Actemra®).
No patients in either trial experienced neurotoxicity, another serious side effect that has been seen in several other trials of CAR T cells and, in one case, led a company to discontinue development of its lead CAR T cell treatment.
Differences in the Therapies, Trials
Unlike most other trials of CAR T cells, including the bluebird trial, patients in the Chinese trial received three smaller doses of the cells rather than a single large dose. Patients received a relatively puny dose on their very first day of therapy, and then two progressively larger doses on days two and 6.
This was done largely for safety reasons, explained Frank Fan, Ph.D., the chief scientific officer for Nanjing Legend. In some cases, if patients were experiencing CRS or any other potentially serious side effect after the 2nd dose, the third dose was not given, Dr. Fan said.
The receptor on the LCAR-B38M CAR T cells is also different from those on most other CAR T cells in development, he continued, in that it can fasten to two different parts of the same antigen. This difference, he believes, may improve its capability to tie to and kill tumor cells.
Interpreting the significance of the Chinese trial results is difficult because of some significant missing details, said Marcela Maus, M.D., Ph.D., director of Cellular Immunotherapy at the Massachusetts General Hospital Cancer Center.
During an abstract discussion session at the meeting where the trial results were introduced, Dr. Maus said more information is still needed on the extent and types of prior therapies received by patients, the criteria used to determine the treatment responses, and the composition of the T cells used in the therapy administered to patients.
In the bluebird trial, not only had all of the patients been strenuously pretreated, Dr. Berdeja said, but almost 30% of patients were resistant to all of the major drug classes used to treat numerous myeloma.
Next Steps
The researchers leading the Nanjing Legend trial are continuing to enroll patients in their trial and are filing the regulatory paperwork with FDA to launch a similar trial in the United States early next year, Dr. Fan said.
The bluebird trial will now proceed to what is known as the dose-expansion stage, Dr. Berdeja explained, using the dose established as most safe and effective in the trial’s initial dose-escalation stage.
CAR T-Cell Therapy for Numerous Myeloma – National Cancer Institute
CAR T Cells: Expanding into Numerous Myeloma
June 12, 2017, by NCI Staff
Before (left) and after (right) PET scans of a patient with numerous myeloma treated with BCMA-targeted CAR T cells.
Results from two early-phase clinical trials suggest that a form of immunotherapy that uses genetically engineered immune cells may be very effective in patients with advanced numerous myeloma.
Both trials used CAR T cells that were engineered to target a protein on myeloma cells called B-cell maturation antigen (BCMA). Albeit most patients in the trials had good responses to the treatment, including many who experienced finish remissions, the length of follow-up for most patients is still limited, researchers from both trials cautioned.
Preliminary findings from the trials were introduced this week at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. One trial was conducted in China and the other in the United States.
Serious side effects, including immune-related effects seen in other CAR T-cell trials, have been limited in the patients treated to date, reported the investigators leading the trials.
Albeit it’s still too early to determine whether these BCMA-targeted CAR T cells will eventually become standard treatments for numerous myeloma, some researchers were encouraged by the early results.
“The science behind [CAR T cells] is getting to be fairly revolutionary,” said Michael Sabel, M.D., of the University of Michigan Comprehensive Cancer Center, during a press briefing on the results of the Chinese trial. But more research is needed, Dr. Sabel continued, to validate these early findings and “to see if we can make this type of therapy accessible to more patients.”
An Emerging Form of Immunotherapy
A form of immunotherapy known as checkpoint inhibition is rapidly becoming an established part of everyday cancer care. The Food and Drug Administration (FDA) has approved checkpoint inhibitors, which are biologic drugs known as humanized monoclonal antibodies, for more types of cancer, including melanoma, lung cancer, lymphoma, and bladder cancer.
And in May, the agency approved the checkpoint inhibitor pembrolizumab (Keytruda®) for use in adults or children with advanced cancer—regardless of their type of cancer—whose tumors have one of two types of genetic alterations.
CAR T cells, on the other arm, use a patient’s own immune cells to treat their disease and are still largely in the clinical development phase.
In this form of therapy, immune cells are collected from a patient’s blood; engineered in the laboratory to produce special receptors called chimeric-antigen receptors, or CARs, that fasten to a specific target on cancer cells (an antigen); grown in the lab until they number in the billions; and then reinfused back into the patient.
Two companies have recently filed approval applications with FDA for their CAR T-cell therapies, one for lymphoma and the other for leukemia.
The CAR T-cell products under development differ from one another in several ways, including the antigen on tumor cells that they are engineered to target. Both of the CAR T-cell products that have been submitted for FDA approval, including one originally developed at NCI, target the CD19 antigen.
CAR T cells that target BCMA are relatively fresh. BCMA-targeted CAR T cells were very first tested in two thousand fourteen at NCI, and several other ongoing trials, in addition to the two reported at ASCO, are testing them.
In blood cells, the expression of BCMA is limited primarily to normal and cancerous plasma cells, explained James Kochenderfer, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research. BCMA is voiced in most patients with numerous myeloma, he added, and it’s not voiced on any nonblood cells.
“The most significant factor in determining what antigen to target with a CAR is to pick one that is not voiced on essential normal tissues,” said Dr. Kochenderfer, senior author of the US-conducted trial. Dr. Kochenderfer’s lab developed the very first BCMA-targeted CAR T cells and, in 2014, he and his colleagues at NCI conducted the very first human trial to test BCMA-targeted CAR T cells.
Many Remissions
In the Chinese trial, which is being funded by Nanjing Legend Biotech, thirty three of the thirty five patients in the investigate went into finish remission within two months of receiving the BCMA-targeted CAR T cells, reported trial investigator Wanhong Zhao, M.D., Ph.D., of Xi’an Jiaotong University. The remaining two patients also had tumor responses, Dr. Zhao said.
Of the nineteen patients who have been followed for at least four months, fourteen have had what is called a stringent accomplish response (sCR)—that is, no evidence of the disease in their bone marrow or other markers of the disease in other tests—to the treatment, which the company is calling LCAR-B38M CAR T cells. Of the fourteen patients with an sCR, the five who have been followed for at least a year still have no signs of cancer, Dr. Zhao said.
Jesus G. Berdeja, M.D., of the Sarah Cannon Research Institute in Tennessee, introduced updated findings from the other trial of BCMA-targeted CAR T cells, which is being funded by bluebird bio, Inc. To date, twenty one patients have been treated on the trial. Dr. Berdeja reported on data from eighteen of these patients. (The remaining three have not been on the trial long enough to be evaluated.)
As is often done in phase I trials, different doses of the CAR T-cell product—which the company is calling bb2121—were tested, with the intention of step by step enhancing the dose if no serious side effects were seen and establishing the best dose to test in larger trials.
Only one of the very first three patients treated at the lowest dose of CAR T cells responded. Beyond this first-level dose, however, the overall response rate was 100%, with 27% of patients experiencing an sCR and the remainder having what is known as a very good partial response (based on widely used and accepted criteria).
“These patients were powerfully pretreated,” Dr. Berdeja said. The median number of prior treatments was seven, he noted; all of the patients in the trial had at least one stem cell transplant, and several have had numerous transplants.
The strong responses in patients who had been so powerfully pretreated are not the only encouraging findings from the trial, Dr. Berdeja said.
“What’s so striking is that the tumor cells are killed so quickly that all the other parameters [of treatment response] lag behind,” he said.
Manageable Side Effects
All patients undergoing CAR T-cell therapy are closely monitored for a known side effect called cytokine release syndrome (CRS), a massive storm of immune signaling cells called cytokines that can be fatal.
Minor cases of CRS were common in both trials, the researchers reported, but were generally brief lived and managed with standard therapies.
Several patients in both trials experienced severe CRS following treatment, which was treated successfully with the drug tocilizumab (Actemra®).
No patients in either trial experienced neurotoxicity, another serious side effect that has been seen in several other trials of CAR T cells and, in one case, led a company to discontinue development of its lead CAR T cell treatment.
Differences in the Therapies, Trials
Unlike most other trials of CAR T cells, including the bluebird trial, patients in the Chinese trial received three smaller doses of the cells rather than a single large dose. Patients received a relatively puny dose on their very first day of therapy, and then two progressively larger doses on days two and 6.
This was done largely for safety reasons, explained Frank Fan, Ph.D., the chief scientific officer for Nanjing Legend. In some cases, if patients were experiencing CRS or any other potentially serious side effect after the 2nd dose, the third dose was not given, Dr. Fan said.
The receptor on the LCAR-B38M CAR T cells is also different from those on most other CAR T cells in development, he continued, in that it can link to two different parts of the same antigen. This difference, he believes, may improve its capability to tie to and kill tumor cells.
Interpreting the significance of the Chinese trial results is difficult because of some significant missing details, said Marcela Maus, M.D., Ph.D., director of Cellular Immunotherapy at the Massachusetts General Hospital Cancer Center.
During an abstract discussion session at the meeting where the trial results were introduced, Dr. Maus said more information is still needed on the extent and types of prior therapies received by patients, the criteria used to determine the treatment responses, and the composition of the T cells used in the therapy administered to patients.
In the bluebird trial, not only had all of the patients been strenuously pretreated, Dr. Berdeja said, but almost 30% of patients were resistant to all of the major drug classes used to treat numerous myeloma.
Next Steps
The researchers leading the Nanjing Legend trial are continuing to enroll patients in their trial and are filing the regulatory paperwork with FDA to launch a similar trial in the United States early next year, Dr. Fan said.
The bluebird trial will now proceed to what is known as the dose-expansion stage, Dr. Berdeja explained, using the dose established as most safe and effective in the trial’s initial dose-escalation stage.
CAR T-Cell Therapy for Numerous Myeloma – National Cancer Institute
CAR T Cells: Expanding into Numerous Myeloma
June 12, 2017, by NCI Staff
Before (left) and after (right) PET scans of a patient with numerous myeloma treated with BCMA-targeted CAR T cells.
Results from two early-phase clinical trials suggest that a form of immunotherapy that uses genetically engineered immune cells may be very effective in patients with advanced numerous myeloma.
Both trials used CAR T cells that were engineered to target a protein on myeloma cells called B-cell maturation antigen (BCMA). Albeit most patients in the trials had good responses to the treatment, including many who experienced accomplish remissions, the length of follow-up for most patients is still limited, researchers from both trials cautioned.
Preliminary findings from the trials were introduced this week at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. One trial was conducted in China and the other in the United States.
Serious side effects, including immune-related effects seen in other CAR T-cell trials, have been limited in the patients treated to date, reported the investigators leading the trials.
Albeit it’s still too early to determine whether these BCMA-targeted CAR T cells will eventually become standard treatments for numerous myeloma, some researchers were encouraged by the early results.
“The science behind [CAR T cells] is getting to be fairly revolutionary,” said Michael Sabel, M.D., of the University of Michigan Comprehensive Cancer Center, during a press briefing on the results of the Chinese trial. But more research is needed, Dr. Sabel continued, to validate these early findings and “to see if we can make this type of therapy accessible to more patients.”
An Emerging Form of Immunotherapy
A form of immunotherapy known as checkpoint inhibition is rapidly becoming an established part of everyday cancer care. The Food and Drug Administration (FDA) has approved checkpoint inhibitors, which are biologic drugs known as humanized monoclonal antibodies, for more types of cancer, including melanoma, lung cancer, lymphoma, and bladder cancer.
And in May, the agency approved the checkpoint inhibitor pembrolizumab (Keytruda®) for use in adults or children with advanced cancer—regardless of their type of cancer—whose tumors have one of two types of genetic alterations.
CAR T cells, on the other palm, use a patient’s own immune cells to treat their disease and are still largely in the clinical development phase.
In this form of therapy, immune cells are collected from a patient’s blood; engineered in the laboratory to produce special receptors called chimeric-antigen receptors, or CARs, that fasten to a specific target on cancer cells (an antigen); grown in the lab until they number in the billions; and then reinfused back into the patient.
Two companies have recently filed approval applications with FDA for their CAR T-cell therapies, one for lymphoma and the other for leukemia.
The CAR T-cell products under development differ from one another in several ways, including the antigen on tumor cells that they are engineered to target. Both of the CAR T-cell products that have been submitted for FDA approval, including one primarily developed at NCI, target the CD19 antigen.
CAR T cells that target BCMA are relatively fresh. BCMA-targeted CAR T cells were very first tested in two thousand fourteen at NCI, and several other ongoing trials, in addition to the two reported at ASCO, are testing them.
In blood cells, the expression of BCMA is limited primarily to normal and cancerous plasma cells, explained James Kochenderfer, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research. BCMA is voiced in most patients with numerous myeloma, he added, and it’s not voiced on any nonblood cells.
“The most significant factor in determining what antigen to target with a CAR is to pick one that is not voiced on essential normal tissues,” said Dr. Kochenderfer, senior author of the US-conducted trial. Dr. Kochenderfer’s lab developed the very first BCMA-targeted CAR T cells and, in 2014, he and his colleagues at NCI conducted the very first human trial to test BCMA-targeted CAR T cells.
Many Remissions
In the Chinese trial, which is being funded by Nanjing Legend Biotech, thirty three of the thirty five patients in the explore went into finish remission within two months of receiving the BCMA-targeted CAR T cells, reported trial investigator Wanhong Zhao, M.D., Ph.D., of Xi’an Jiaotong University. The remaining two patients also had tumor responses, Dr. Zhao said.
Of the nineteen patients who have been followed for at least four months, fourteen have had what is called a stringent accomplish response (sCR)—that is, no evidence of the disease in their bone marrow or other markers of the disease in other tests—to the treatment, which the company is calling LCAR-B38M CAR T cells. Of the fourteen patients with an sCR, the five who have been followed for at least a year still have no signs of cancer, Dr. Zhao said.
Jesus G. Berdeja, M.D., of the Sarah Cannon Research Institute in Tennessee, introduced updated findings from the other trial of BCMA-targeted CAR T cells, which is being funded by bluebird bio, Inc. To date, twenty one patients have been treated on the trial. Dr. Berdeja reported on data from eighteen of these patients. (The remaining three have not been on the trial long enough to be evaluated.)
As is often done in phase I trials, different doses of the CAR T-cell product—which the company is calling bb2121—were tested, with the intention of little by little enlargening the dose if no serious side effects were seen and establishing the best dose to test in larger trials.
Only one of the very first three patients treated at the lowest dose of CAR T cells responded. Beyond this first-level dose, however, the overall response rate was 100%, with 27% of patients experiencing an sCR and the remainder having what is known as a very good partial response (based on widely used and accepted criteria).
“These patients were intensely pretreated,” Dr. Berdeja said. The median number of prior treatments was seven, he noted; all of the patients in the trial had at least one stem cell transplant, and several have had numerous transplants.
The strong responses in patients who had been so strenuously pretreated are not the only encouraging findings from the trial, Dr. Berdeja said.
“What’s so striking is that the tumor cells are killed so quickly that all the other parameters [of treatment response] lag behind,” he said.
Manageable Side Effects
All patients undergoing CAR T-cell therapy are closely monitored for a known side effect called cytokine release syndrome (CRS), a massive storm of immune signaling cells called cytokines that can be fatal.
Minor cases of CRS were common in both trials, the researchers reported, but were generally brief lived and managed with standard therapies.
Several patients in both trials experienced severe CRS following treatment, which was treated successfully with the drug tocilizumab (Actemra®).
No patients in either trial experienced neurotoxicity, another serious side effect that has been seen in several other trials of CAR T cells and, in one case, led a company to discontinue development of its lead CAR T cell treatment.
Differences in the Therapies, Trials
Unlike most other trials of CAR T cells, including the bluebird trial, patients in the Chinese trial received three smaller doses of the cells rather than a single large dose. Patients received a relatively petite dose on their very first day of therapy, and then two progressively larger doses on days two and 6.
This was done largely for safety reasons, explained Frank Fan, Ph.D., the chief scientific officer for Nanjing Legend. In some cases, if patients were experiencing CRS or any other potentially serious side effect after the 2nd dose, the third dose was not given, Dr. Fan said.
The receptor on the LCAR-B38M CAR T cells is also different from those on most other CAR T cells in development, he continued, in that it can fasten to two different parts of the same antigen. This difference, he believes, may improve its capability to truss to and kill tumor cells.
Interpreting the significance of the Chinese trial results is difficult because of some significant missing details, said Marcela Maus, M.D., Ph.D., director of Cellular Immunotherapy at the Massachusetts General Hospital Cancer Center.
During an abstract discussion session at the meeting where the trial results were introduced, Dr. Maus said more information is still needed on the extent and types of prior therapies received by patients, the criteria used to determine the treatment responses, and the composition of the T cells used in the therapy administered to patients.
In the bluebird trial, not only had all of the patients been powerfully pretreated, Dr. Berdeja said, but almost 30% of patients were resistant to all of the major drug classes used to treat numerous myeloma.
Next Steps
The researchers leading the Nanjing Legend trial are continuing to enroll patients in their trial and are filing the regulatory paperwork with FDA to launch a similar trial in the United States early next year, Dr. Fan said.
The bluebird trial will now proceed to what is known as the dose-expansion stage, Dr. Berdeja explained, using the dose established as most safe and effective in the trial’s initial dose-escalation stage.
CAR T-Cell Therapy for Numerous Myeloma – National Cancer Institute
CAR T Cells: Expanding into Numerous Myeloma
June 12, 2017, by NCI Staff
Before (left) and after (right) PET scans of a patient with numerous myeloma treated with BCMA-targeted CAR T cells.
Results from two early-phase clinical trials suggest that a form of immunotherapy that uses genetically engineered immune cells may be very effective in patients with advanced numerous myeloma.
Both trials used CAR T cells that were engineered to target a protein on myeloma cells called B-cell maturation antigen (BCMA). Albeit most patients in the trials had good responses to the treatment, including many who experienced accomplish remissions, the length of follow-up for most patients is still limited, researchers from both trials cautioned.
Preliminary findings from the trials were introduced this week at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. One trial was conducted in China and the other in the United States.
Serious side effects, including immune-related effects seen in other CAR T-cell trials, have been limited in the patients treated to date, reported the investigators leading the trials.
Albeit it’s still too early to determine whether these BCMA-targeted CAR T cells will eventually become standard treatments for numerous myeloma, some researchers were encouraged by the early results.
“The science behind [CAR T cells] is getting to be fairly revolutionary,” said Michael Sabel, M.D., of the University of Michigan Comprehensive Cancer Center, during a press briefing on the results of the Chinese trial. But more research is needed, Dr. Sabel continued, to validate these early findings and “to see if we can make this type of therapy accessible to more patients.”
An Emerging Form of Immunotherapy
A form of immunotherapy known as checkpoint inhibition is rapidly becoming an established part of everyday cancer care. The Food and Drug Administration (FDA) has approved checkpoint inhibitors, which are biologic drugs known as humanized monoclonal antibodies, for more types of cancer, including melanoma, lung cancer, lymphoma, and bladder cancer.
And in May, the agency approved the checkpoint inhibitor pembrolizumab (Keytruda®) for use in adults or children with advanced cancer—regardless of their type of cancer—whose tumors have one of two types of genetic alterations.
CAR T cells, on the other arm, use a patient’s own immune cells to treat their disease and are still largely in the clinical development phase.
In this form of therapy, immune cells are collected from a patient’s blood; engineered in the laboratory to produce special receptors called chimeric-antigen receptors, or CARs, that fasten to a specific target on cancer cells (an antigen); grown in the lab until they number in the billions; and then reinfused back into the patient.
Two companies have recently filed approval applications with FDA for their CAR T-cell therapies, one for lymphoma and the other for leukemia.
The CAR T-cell products under development differ from one another in several ways, including the antigen on tumor cells that they are engineered to target. Both of the CAR T-cell products that have been submitted for FDA approval, including one originally developed at NCI, target the CD19 antigen.
CAR T cells that target BCMA are relatively fresh. BCMA-targeted CAR T cells were very first tested in two thousand fourteen at NCI, and several other ongoing trials, in addition to the two reported at ASCO, are testing them.
In blood cells, the expression of BCMA is limited primarily to normal and cancerous plasma cells, explained James Kochenderfer, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research. BCMA is voiced in most patients with numerous myeloma, he added, and it’s not voiced on any nonblood cells.
“The most significant factor in determining what antigen to target with a CAR is to pick one that is not voiced on essential normal tissues,” said Dr. Kochenderfer, senior author of the US-conducted trial. Dr. Kochenderfer’s lab developed the very first BCMA-targeted CAR T cells and, in 2014, he and his colleagues at NCI conducted the very first human trial to test BCMA-targeted CAR T cells.
Many Remissions
In the Chinese trial, which is being funded by Nanjing Legend Biotech, thirty three of the thirty five patients in the explore went into finish remission within two months of receiving the BCMA-targeted CAR T cells, reported trial investigator Wanhong Zhao, M.D., Ph.D., of Xi’an Jiaotong University. The remaining two patients also had tumor responses, Dr. Zhao said.
Of the nineteen patients who have been followed for at least four months, fourteen have had what is called a stringent accomplish response (sCR)—that is, no evidence of the disease in their bone marrow or other markers of the disease in other tests—to the treatment, which the company is calling LCAR-B38M CAR T cells. Of the fourteen patients with an sCR, the five who have been followed for at least a year still have no signs of cancer, Dr. Zhao said.
Jesus G. Berdeja, M.D., of the Sarah Cannon Research Institute in Tennessee, introduced updated findings from the other trial of BCMA-targeted CAR T cells, which is being funded by bluebird bio, Inc. To date, twenty one patients have been treated on the trial. Dr. Berdeja reported on data from eighteen of these patients. (The remaining three have not been on the trial long enough to be evaluated.)
As is often done in phase I trials, different doses of the CAR T-cell product—which the company is calling bb2121—were tested, with the intention of step by step enhancing the dose if no serious side effects were seen and establishing the best dose to test in larger trials.
Only one of the very first three patients treated at the lowest dose of CAR T cells responded. Beyond this first-level dose, however, the overall response rate was 100%, with 27% of patients experiencing an sCR and the remainder having what is known as a very good partial response (based on widely used and accepted criteria).
“These patients were intensely pretreated,” Dr. Berdeja said. The median number of prior treatments was seven, he noted; all of the patients in the trial had at least one stem cell transplant, and several have had numerous transplants.
The strong responses in patients who had been so powerfully pretreated are not the only encouraging findings from the trial, Dr. Berdeja said.
“What’s so striking is that the tumor cells are killed so quickly that all the other parameters [of treatment response] lag behind,” he said.
Manageable Side Effects
All patients undergoing CAR T-cell therapy are closely monitored for a known side effect called cytokine release syndrome (CRS), a massive storm of immune signaling cells called cytokines that can be fatal.
Minor cases of CRS were common in both trials, the researchers reported, but were generally brief lived and managed with standard therapies.
Several patients in both trials experienced severe CRS following treatment, which was treated successfully with the drug tocilizumab (Actemra®).
No patients in either trial experienced neurotoxicity, another serious side effect that has been seen in several other trials of CAR T cells and, in one case, led a company to discontinue development of its lead CAR T cell treatment.
Differences in the Therapies, Trials
Unlike most other trials of CAR T cells, including the bluebird trial, patients in the Chinese trial received three smaller doses of the cells rather than a single large dose. Patients received a relatively puny dose on their very first day of therapy, and then two progressively larger doses on days two and 6.
This was done largely for safety reasons, explained Frank Fan, Ph.D., the chief scientific officer for Nanjing Legend. In some cases, if patients were experiencing CRS or any other potentially serious side effect after the 2nd dose, the third dose was not given, Dr. Fan said.
The receptor on the LCAR-B38M CAR T cells is also different from those on most other CAR T cells in development, he continued, in that it can fasten to two different parts of the same antigen. This difference, he believes, may improve its capability to truss to and kill tumor cells.
Interpreting the significance of the Chinese trial results is difficult because of some significant missing details, said Marcela Maus, M.D., Ph.D., director of Cellular Immunotherapy at the Massachusetts General Hospital Cancer Center.
During an abstract discussion session at the meeting where the trial results were introduced, Dr. Maus said more information is still needed on the extent and types of prior therapies received by patients, the criteria used to determine the treatment responses, and the composition of the T cells used in the therapy administered to patients.
In the bluebird trial, not only had all of the patients been powerfully pretreated, Dr. Berdeja said, but almost 30% of patients were resistant to all of the major drug classes used to treat numerous myeloma.
Next Steps
The researchers leading the Nanjing Legend trial are continuing to enroll patients in their trial and are filing the regulatory paperwork with FDA to launch a similar trial in the United States early next year, Dr. Fan said.
The bluebird trial will now proceed to what is known as the dose-expansion stage, Dr. Berdeja explained, using the dose established as most safe and effective in the trial’s initial dose-escalation stage.
CAR T-Cell Therapy for Numerous Myeloma – National Cancer Institute
CAR T Cells: Expanding into Numerous Myeloma
June 12, 2017, by NCI Staff
Before (left) and after (right) PET scans of a patient with numerous myeloma treated with BCMA-targeted CAR T cells.
Results from two early-phase clinical trials suggest that a form of immunotherapy that uses genetically engineered immune cells may be very effective in patients with advanced numerous myeloma.
Both trials used CAR T cells that were engineered to target a protein on myeloma cells called B-cell maturation antigen (BCMA). Albeit most patients in the trials had good responses to the treatment, including many who experienced finish remissions, the length of follow-up for most patients is still limited, researchers from both trials cautioned.
Preliminary findings from the trials were introduced this week at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. One trial was conducted in China and the other in the United States.
Serious side effects, including immune-related effects seen in other CAR T-cell trials, have been limited in the patients treated to date, reported the investigators leading the trials.
Albeit it’s still too early to determine whether these BCMA-targeted CAR T cells will eventually become standard treatments for numerous myeloma, some researchers were encouraged by the early results.
“The science behind [CAR T cells] is getting to be fairly revolutionary,” said Michael Sabel, M.D., of the University of Michigan Comprehensive Cancer Center, during a press briefing on the results of the Chinese trial. But more research is needed, Dr. Sabel continued, to validate these early findings and “to see if we can make this type of therapy accessible to more patients.”
An Emerging Form of Immunotherapy
A form of immunotherapy known as checkpoint inhibition is rapidly becoming an established part of everyday cancer care. The Food and Drug Administration (FDA) has approved checkpoint inhibitors, which are biologic drugs known as humanized monoclonal antibodies, for more types of cancer, including melanoma, lung cancer, lymphoma, and bladder cancer.
And in May, the agency approved the checkpoint inhibitor pembrolizumab (Keytruda®) for use in adults or children with advanced cancer—regardless of their type of cancer—whose tumors have one of two types of genetic alterations.
CAR T cells, on the other arm, use a patient’s own immune cells to treat their disease and are still largely in the clinical development phase.
In this form of therapy, immune cells are collected from a patient’s blood; engineered in the laboratory to produce special receptors called chimeric-antigen receptors, or CARs, that fasten to a specific target on cancer cells (an antigen); grown in the lab until they number in the billions; and then reinfused back into the patient.
Two companies have recently filed approval applications with FDA for their CAR T-cell therapies, one for lymphoma and the other for leukemia.
The CAR T-cell products under development differ from one another in several ways, including the antigen on tumor cells that they are engineered to target. Both of the CAR T-cell products that have been submitted for FDA approval, including one originally developed at NCI, target the CD19 antigen.
CAR T cells that target BCMA are relatively fresh. BCMA-targeted CAR T cells were very first tested in two thousand fourteen at NCI, and several other ongoing trials, in addition to the two reported at ASCO, are testing them.
In blood cells, the expression of BCMA is limited primarily to normal and cancerous plasma cells, explained James Kochenderfer, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research. BCMA is voiced in most patients with numerous myeloma, he added, and it’s not voiced on any nonblood cells.
“The most significant factor in determining what antigen to target with a CAR is to pick one that is not voiced on essential normal tissues,” said Dr. Kochenderfer, senior author of the US-conducted trial. Dr. Kochenderfer’s lab developed the very first BCMA-targeted CAR T cells and, in 2014, he and his colleagues at NCI conducted the very first human trial to test BCMA-targeted CAR T cells.
Many Remissions
In the Chinese trial, which is being funded by Nanjing Legend Biotech, thirty three of the thirty five patients in the examine went into accomplish remission within two months of receiving the BCMA-targeted CAR T cells, reported trial investigator Wanhong Zhao, M.D., Ph.D., of Xi’an Jiaotong University. The remaining two patients also had tumor responses, Dr. Zhao said.
Of the nineteen patients who have been followed for at least four months, fourteen have had what is called a stringent finish response (sCR)—that is, no evidence of the disease in their bone marrow or other markers of the disease in other tests—to the treatment, which the company is calling LCAR-B38M CAR T cells. Of the fourteen patients with an sCR, the five who have been followed for at least a year still have no signs of cancer, Dr. Zhao said.
Jesus G. Berdeja, M.D., of the Sarah Cannon Research Institute in Tennessee, introduced updated findings from the other trial of BCMA-targeted CAR T cells, which is being funded by bluebird bio, Inc. To date, twenty one patients have been treated on the trial. Dr. Berdeja reported on data from eighteen of these patients. (The remaining three have not been on the trial long enough to be evaluated.)
As is often done in phase I trials, different doses of the CAR T-cell product—which the company is calling bb2121—were tested, with the intention of little by little enlargening the dose if no serious side effects were seen and establishing the best dose to test in larger trials.
Only one of the very first three patients treated at the lowest dose of CAR T cells responded. Beyond this first-level dose, however, the overall response rate was 100%, with 27% of patients experiencing an sCR and the remainder having what is known as a very good partial response (based on widely used and accepted criteria).
“These patients were powerfully pretreated,” Dr. Berdeja said. The median number of prior treatments was seven, he noted; all of the patients in the trial had at least one stem cell transplant, and several have had numerous transplants.
The strong responses in patients who had been so strongly pretreated are not the only encouraging findings from the trial, Dr. Berdeja said.
“What’s so striking is that the tumor cells are killed so quickly that all the other parameters [of treatment response] lag behind,” he said.
Manageable Side Effects
All patients undergoing CAR T-cell therapy are closely monitored for a known side effect called cytokine release syndrome (CRS), a massive storm of immune signaling cells called cytokines that can be fatal.
Minor cases of CRS were common in both trials, the researchers reported, but were generally brief lived and managed with standard therapies.
Several patients in both trials experienced severe CRS following treatment, which was treated successfully with the drug tocilizumab (Actemra®).
No patients in either trial experienced neurotoxicity, another serious side effect that has been seen in several other trials of CAR T cells and, in one case, led a company to discontinue development of its lead CAR T cell treatment.
Differences in the Therapies, Trials
Unlike most other trials of CAR T cells, including the bluebird trial, patients in the Chinese trial received three smaller doses of the cells rather than a single large dose. Patients received a relatively puny dose on their very first day of therapy, and then two progressively larger doses on days two and 6.
This was done largely for safety reasons, explained Frank Fan, Ph.D., the chief scientific officer for Nanjing Legend. In some cases, if patients were experiencing CRS or any other potentially serious side effect after the 2nd dose, the third dose was not given, Dr. Fan said.
The receptor on the LCAR-B38M CAR T cells is also different from those on most other CAR T cells in development, he continued, in that it can fasten to two different parts of the same antigen. This difference, he believes, may improve its capability to tie to and kill tumor cells.
Interpreting the significance of the Chinese trial results is difficult because of some significant missing details, said Marcela Maus, M.D., Ph.D., director of Cellular Immunotherapy at the Massachusetts General Hospital Cancer Center.
During an abstract discussion session at the meeting where the trial results were introduced, Dr. Maus said more information is still needed on the extent and types of prior therapies received by patients, the criteria used to determine the treatment responses, and the composition of the T cells used in the therapy administered to patients.
In the bluebird trial, not only had all of the patients been intensely pretreated, Dr. Berdeja said, but almost 30% of patients were resistant to all of the major drug classes used to treat numerous myeloma.
Next Steps
The researchers leading the Nanjing Legend trial are continuing to enroll patients in their trial and are filing the regulatory paperwork with FDA to launch a similar trial in the United States early next year, Dr. Fan said.
The bluebird trial will now proceed to what is known as the dose-expansion stage, Dr. Berdeja explained, using the dose established as most safe and effective in the trial’s initial dose-escalation stage.
CAR T-Cell Therapy for Numerous Myeloma – National Cancer Institute
CAR T Cells: Expanding into Numerous Myeloma
June 12, 2017, by NCI Staff
Before (left) and after (right) PET scans of a patient with numerous myeloma treated with BCMA-targeted CAR T cells.
Results from two early-phase clinical trials suggest that a form of immunotherapy that uses genetically engineered immune cells may be very effective in patients with advanced numerous myeloma.
Both trials used CAR T cells that were engineered to target a protein on myeloma cells called B-cell maturation antigen (BCMA). Albeit most patients in the trials had good responses to the treatment, including many who experienced accomplish remissions, the length of follow-up for most patients is still limited, researchers from both trials cautioned.
Preliminary findings from the trials were introduced this week at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. One trial was conducted in China and the other in the United States.
Serious side effects, including immune-related effects seen in other CAR T-cell trials, have been limited in the patients treated to date, reported the investigators leading the trials.
Albeit it’s still too early to determine whether these BCMA-targeted CAR T cells will eventually become standard treatments for numerous myeloma, some researchers were encouraged by the early results.
“The science behind [CAR T cells] is getting to be fairly revolutionary,” said Michael Sabel, M.D., of the University of Michigan Comprehensive Cancer Center, during a press briefing on the results of the Chinese trial. But more research is needed, Dr. Sabel continued, to validate these early findings and “to see if we can make this type of therapy accessible to more patients.”
An Emerging Form of Immunotherapy
A form of immunotherapy known as checkpoint inhibition is rapidly becoming an established part of everyday cancer care. The Food and Drug Administration (FDA) has approved checkpoint inhibitors, which are biologic drugs known as humanized monoclonal antibodies, for more types of cancer, including melanoma, lung cancer, lymphoma, and bladder cancer.
And in May, the agency approved the checkpoint inhibitor pembrolizumab (Keytruda®) for use in adults or children with advanced cancer—regardless of their type of cancer—whose tumors have one of two types of genetic alterations.
CAR T cells, on the other arm, use a patient’s own immune cells to treat their disease and are still largely in the clinical development phase.
In this form of therapy, immune cells are collected from a patient’s blood; engineered in the laboratory to produce special receptors called chimeric-antigen receptors, or CARs, that fasten to a specific target on cancer cells (an antigen); grown in the lab until they number in the billions; and then reinfused back into the patient.
Two companies have recently filed approval applications with FDA for their CAR T-cell therapies, one for lymphoma and the other for leukemia.
The CAR T-cell products under development differ from one another in several ways, including the antigen on tumor cells that they are engineered to target. Both of the CAR T-cell products that have been submitted for FDA approval, including one originally developed at NCI, target the CD19 antigen.
CAR T cells that target BCMA are relatively fresh. BCMA-targeted CAR T cells were very first tested in two thousand fourteen at NCI, and several other ongoing trials, in addition to the two reported at ASCO, are testing them.
In blood cells, the expression of BCMA is limited primarily to normal and cancerous plasma cells, explained James Kochenderfer, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research. BCMA is voiced in most patients with numerous myeloma, he added, and it’s not voiced on any nonblood cells.
“The most significant factor in determining what antigen to target with a CAR is to pick one that is not voiced on essential normal tissues,” said Dr. Kochenderfer, senior author of the US-conducted trial. Dr. Kochenderfer’s lab developed the very first BCMA-targeted CAR T cells and, in 2014, he and his colleagues at NCI conducted the very first human trial to test BCMA-targeted CAR T cells.
Many Remissions
In the Chinese trial, which is being funded by Nanjing Legend Biotech, thirty three of the thirty five patients in the investigate went into accomplish remission within two months of receiving the BCMA-targeted CAR T cells, reported trial investigator Wanhong Zhao, M.D., Ph.D., of Xi’an Jiaotong University. The remaining two patients also had tumor responses, Dr. Zhao said.
Of the nineteen patients who have been followed for at least four months, fourteen have had what is called a stringent accomplish response (sCR)—that is, no evidence of the disease in their bone marrow or other markers of the disease in other tests—to the treatment, which the company is calling LCAR-B38M CAR T cells. Of the fourteen patients with an sCR, the five who have been followed for at least a year still have no signs of cancer, Dr. Zhao said.
Jesus G. Berdeja, M.D., of the Sarah Cannon Research Institute in Tennessee, introduced updated findings from the other trial of BCMA-targeted CAR T cells, which is being funded by bluebird bio, Inc. To date, twenty one patients have been treated on the trial. Dr. Berdeja reported on data from eighteen of these patients. (The remaining three have not been on the trial long enough to be evaluated.)
As is often done in phase I trials, different doses of the CAR T-cell product—which the company is calling bb2121—were tested, with the intention of step by step enhancing the dose if no serious side effects were seen and establishing the best dose to test in larger trials.
Only one of the very first three patients treated at the lowest dose of CAR T cells responded. Beyond this first-level dose, however, the overall response rate was 100%, with 27% of patients experiencing an sCR and the remainder having what is known as a very good partial response (based on widely used and accepted criteria).
“These patients were intensely pretreated,” Dr. Berdeja said. The median number of prior treatments was seven, he noted; all of the patients in the trial had at least one stem cell transplant, and several have had numerous transplants.
The strong responses in patients who had been so intensely pretreated are not the only encouraging findings from the trial, Dr. Berdeja said.
“What’s so striking is that the tumor cells are killed so quickly that all the other parameters [of treatment response] lag behind,” he said.
Manageable Side Effects
All patients undergoing CAR T-cell therapy are closely monitored for a known side effect called cytokine release syndrome (CRS), a massive storm of immune signaling cells called cytokines that can be fatal.
Minor cases of CRS were common in both trials, the researchers reported, but were generally brief lived and managed with standard therapies.
Several patients in both trials experienced severe CRS following treatment, which was treated successfully with the drug tocilizumab (Actemra®).
No patients in either trial experienced neurotoxicity, another serious side effect that has been seen in several other trials of CAR T cells and, in one case, led a company to discontinue development of its lead CAR T cell treatment.
Differences in the Therapies, Trials
Unlike most other trials of CAR T cells, including the bluebird trial, patients in the Chinese trial received three smaller doses of the cells rather than a single large dose. Patients received a relatively puny dose on their very first day of therapy, and then two progressively larger doses on days two and 6.
This was done largely for safety reasons, explained Frank Fan, Ph.D., the chief scientific officer for Nanjing Legend. In some cases, if patients were experiencing CRS or any other potentially serious side effect after the 2nd dose, the third dose was not given, Dr. Fan said.
The receptor on the LCAR-B38M CAR T cells is also different from those on most other CAR T cells in development, he continued, in that it can fasten to two different parts of the same antigen. This difference, he believes, may improve its capability to tie to and kill tumor cells.
Interpreting the significance of the Chinese trial results is difficult because of some significant missing details, said Marcela Maus, M.D., Ph.D., director of Cellular Immunotherapy at the Massachusetts General Hospital Cancer Center.
During an abstract discussion session at the meeting where the trial results were introduced, Dr. Maus said more information is still needed on the extent and types of prior therapies received by patients, the criteria used to determine the treatment responses, and the composition of the T cells used in the therapy administered to patients.
In the bluebird trial, not only had all of the patients been strenuously pretreated, Dr. Berdeja said, but almost 30% of patients were resistant to all of the major drug classes used to treat numerous myeloma.
Next Steps
The researchers leading the Nanjing Legend trial are continuing to enroll patients in their trial and are filing the regulatory paperwork with FDA to launch a similar trial in the United States early next year, Dr. Fan said.
The bluebird trial will now proceed to what is known as the dose-expansion stage, Dr. Berdeja explained, using the dose established as most safe and effective in the trial’s initial dose-escalation stage.
CAR T-Cell Therapy for Numerous Myeloma – National Cancer Institute
CAR T Cells: Expanding into Numerous Myeloma
June 12, 2017, by NCI Staff
Before (left) and after (right) PET scans of a patient with numerous myeloma treated with BCMA-targeted CAR T cells.
Results from two early-phase clinical trials suggest that a form of immunotherapy that uses genetically engineered immune cells may be very effective in patients with advanced numerous myeloma.
Both trials used CAR T cells that were engineered to target a protein on myeloma cells called B-cell maturation antigen (BCMA). Albeit most patients in the trials had good responses to the treatment, including many who experienced accomplish remissions, the length of follow-up for most patients is still limited, researchers from both trials cautioned.
Preliminary findings from the trials were introduced this week at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. One trial was conducted in China and the other in the United States.
Serious side effects, including immune-related effects seen in other CAR T-cell trials, have been limited in the patients treated to date, reported the investigators leading the trials.
Albeit it’s still too early to determine whether these BCMA-targeted CAR T cells will eventually become standard treatments for numerous myeloma, some researchers were encouraged by the early results.
“The science behind [CAR T cells] is getting to be fairly revolutionary,” said Michael Sabel, M.D., of the University of Michigan Comprehensive Cancer Center, during a press briefing on the results of the Chinese trial. But more research is needed, Dr. Sabel continued, to validate these early findings and “to see if we can make this type of therapy accessible to more patients.”
An Emerging Form of Immunotherapy
A form of immunotherapy known as checkpoint inhibition is rapidly becoming an established part of everyday cancer care. The Food and Drug Administration (FDA) has approved checkpoint inhibitors, which are biologic drugs known as humanized monoclonal antibodies, for more types of cancer, including melanoma, lung cancer, lymphoma, and bladder cancer.
And in May, the agency approved the checkpoint inhibitor pembrolizumab (Keytruda®) for use in adults or children with advanced cancer—regardless of their type of cancer—whose tumors have one of two types of genetic alterations.
CAR T cells, on the other forearm, use a patient’s own immune cells to treat their disease and are still largely in the clinical development phase.
In this form of therapy, immune cells are collected from a patient’s blood; engineered in the laboratory to produce special receptors called chimeric-antigen receptors, or CARs, that fasten to a specific target on cancer cells (an antigen); grown in the lab until they number in the billions; and then reinfused back into the patient.
Two companies have recently filed approval applications with FDA for their CAR T-cell therapies, one for lymphoma and the other for leukemia.
The CAR T-cell products under development differ from one another in several ways, including the antigen on tumor cells that they are engineered to target. Both of the CAR T-cell products that have been submitted for FDA approval, including one originally developed at NCI, target the CD19 antigen.
CAR T cells that target BCMA are relatively fresh. BCMA-targeted CAR T cells were very first tested in two thousand fourteen at NCI, and several other ongoing trials, in addition to the two reported at ASCO, are testing them.
In blood cells, the expression of BCMA is limited primarily to normal and cancerous plasma cells, explained James Kochenderfer, M.D., of the Experimental Transplantation and Immunology Branch in NCI’s Center for Cancer Research. BCMA is voiced in most patients with numerous myeloma, he added, and it’s not voiced on any nonblood cells.
“The most significant factor in determining what antigen to target with a CAR is to pick one that is not voiced on essential normal tissues,” said Dr. Kochenderfer, senior author of the US-conducted trial. Dr. Kochenderfer’s lab developed the very first BCMA-targeted CAR T cells and, in 2014, he and his colleagues at NCI conducted the very first human trial to test BCMA-targeted CAR T cells.
Many Remissions
In the Chinese trial, which is being funded by Nanjing Legend Biotech, thirty three of the thirty five patients in the examine went into accomplish remission within two months of receiving the BCMA-targeted CAR T cells, reported trial investigator Wanhong Zhao, M.D., Ph.D., of Xi’an Jiaotong University. The remaining two patients also had tumor responses, Dr. Zhao said.
Of the nineteen patients who have been followed for at least four months, fourteen have had what is called a stringent accomplish response (sCR)—that is, no evidence of the disease in their bone marrow or other markers of the disease in other tests—to the treatment, which the company is calling LCAR-B38M CAR T cells. Of the fourteen patients with an sCR, the five who have been followed for at least a year still have no signs of cancer, Dr. Zhao said.
Jesus G. Berdeja, M.D., of the Sarah Cannon Research Institute in Tennessee, introduced updated findings from the other trial of BCMA-targeted CAR T cells, which is being funded by bluebird bio, Inc. To date, twenty one patients have been treated on the trial. Dr. Berdeja reported on data from eighteen of these patients. (The remaining three have not been on the trial long enough to be evaluated.)
As is often done in phase I trials, different doses of the CAR T-cell product—which the company is calling bb2121—were tested, with the intention of step by step enlargening the dose if no serious side effects were seen and establishing the best dose to test in larger trials.
Only one of the very first three patients treated at the lowest dose of CAR T cells responded. Beyond this first-level dose, however, the overall response rate was 100%, with 27% of patients experiencing an sCR and the remainder having what is known as a very good partial response (based on widely used and accepted criteria).
“These patients were strenuously pretreated,” Dr. Berdeja said. The median number of prior treatments was seven, he noted; all of the patients in the trial had at least one stem cell transplant, and several have had numerous transplants.
The strong responses in patients who had been so strenuously pretreated are not the only encouraging findings from the trial, Dr. Berdeja said.
“What’s so striking is that the tumor cells are killed so quickly that all the other parameters [of treatment response] lag behind,” he said.
Manageable Side Effects
All patients undergoing CAR T-cell therapy are closely monitored for a known side effect called cytokine release syndrome (CRS), a massive storm of immune signaling cells called cytokines that can be fatal.
Minor cases of CRS were common in both trials, the researchers reported, but were generally brief lived and managed with standard therapies.
Several patients in both trials experienced severe CRS following treatment, which was treated successfully with the drug tocilizumab (Actemra®).
No patients in either trial experienced neurotoxicity, another serious side effect that has been seen in several other trials of CAR T cells and, in one case, led a company to discontinue development of its lead CAR T cell treatment.
Differences in the Therapies, Trials
Unlike most other trials of CAR T cells, including the bluebird trial, patients in the Chinese trial received three smaller doses of the cells rather than a single large dose. Patients received a relatively petite dose on their very first day of therapy, and then two progressively larger doses on days two and 6.
This was done largely for safety reasons, explained Frank Fan, Ph.D., the chief scientific officer for Nanjing Legend. In some cases, if patients were experiencing CRS or any other potentially serious side effect after the 2nd dose, the third dose was not given, Dr. Fan said.
The receptor on the LCAR-B38M CAR T cells is also different from those on most other CAR T cells in development, he continued, in that it can fasten to two different parts of the same antigen. This difference, he believes, may improve its capability to tie to and kill tumor cells.
Interpreting the significance of the Chinese trial results is difficult because of some significant missing details, said Marcela Maus, M.D., Ph.D., director of Cellular Immunotherapy at the Massachusetts General Hospital Cancer Center.
During an abstract discussion session at the meeting where the trial results were introduced, Dr. Maus said more information is still needed on the extent and types of prior therapies received by patients, the criteria used to determine the treatment responses, and the composition of the T cells used in the therapy administered to patients.
In the bluebird trial, not only had all of the patients been strongly pretreated, Dr. Berdeja said, but almost 30% of patients were resistant to all of the major drug classes used to treat numerous myeloma.
Next Steps
The researchers leading the Nanjing Legend trial are continuing to enroll patients in their trial and are filing the regulatory paperwork with FDA to launch a similar trial in the United States early next year, Dr. Fan said.
The bluebird trial will now proceed to what is known as the dose-expansion stage, Dr. Berdeja explained, using the dose established as most safe and effective in the trial’s initial dose-escalation stage.